Abstract
Lenalidomide maintenance therapy, initiated around day 100 after autologous stem cell transplantation (ASCT), has been associated a significantly longer time to disease progression and significantly improved overall survival (OS) among patients with multiple myeloma (MM) (McCarthy et al. NEJM 2012). However, the impact of maintenance therapy on the nature of MM relapse is largely unknown. Generally relapse can be categorized in two broad categories as symptomatic, or biochemical relapse. Symptomatic relapse is characterized mostly by high calcium, renal failure, anemia and bony lesions, i.e., CRAB criteria. Biochemical relapse is defined by a resurgence of secretory function of malignant plasma cells without CRAB criteria. We hypothesized that long-term continuous exposure to lenalidomide induces crucial changes in the myeloma microenvironment leading to a discordance between the resurgence of secretory function and the recurrence of aggressive behavior of myeloma cells leading to a higher frequency of biochemical. To define the impact of maintenance therapy on the pattern of disease recurrence and determinants of a more aggressive or indolent relapse course, we retrospectively analyzed two cohorts of MM patients that underwent ASCT.
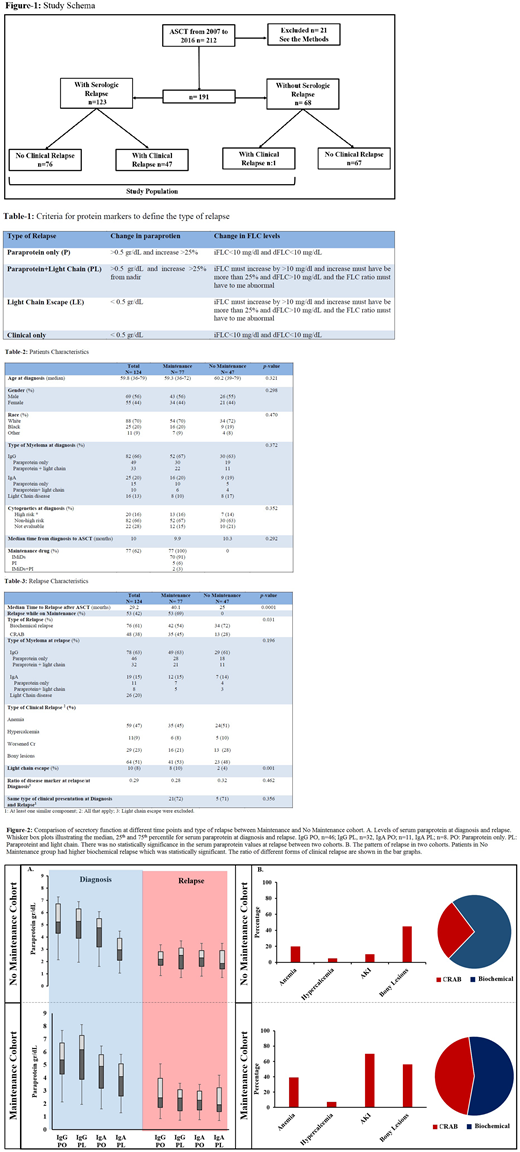
Methods: All MM patients with measurable disease who received ASCT at the University Hospital, Cleveland, OH from 2007 to 2016 were reviewed (Fig. 1). Patients that had received an allogeneic transplant or tandem ASCT, those transplanted more than one year after induction therapy, and those that died during the first three months post-transplantation were excluded. Baseline and treatment characteristics, response status, and monitoring data prior to relapse were extracted. If the serologic relapse had coincided with clinical relapse, defined as per CRAB criteria, patient was categorized as clinical relapse, and otherwise as biochemical relapse. Type of MM was categorized as paraprotein only (PO), paraprotein and light chain (PL), light chain escape (LE) and clinical only (CO) relapse (Table 1). Baseline and treatment characteristics are detailed in Table 2. The univariate association between the type of relapse and key patient and clinical characteristics were assessed using t-test or Fisher's exact test as appropriate.
Results: Study population included 124 pts who relapsed after ASCT (Fig. 1), divided in two cohorts based on history of receiving any maintenance therapy or no maintenance (77 vs. 47 patients, respectively). 21 pts were excluded based on the above criteria. Median age was 59.8 years old (range: 36-79). The median time from diagnosis to ASCT was 10 months. There was only one CO relapse in the maintenance cohort. There was no difference in rate of high risk disease between two cohorts. The maintenance agents included immunomodulatory drugs in 70 pts (91%), proteasome inhibitors in 5 pts (6%) and a combination of both in 2 pts (3%). PFS was 40.1 months in the maintenance group vs. 29.2 months in the no maintenance group (p=0.0001). There was no statistically significant difference in serum paraprotein values at diagnosis or relapse between the two cohorts (Fig. 1A). LCE was more common in the maintenance group. Thirteen patients (28%) in the no maintenance cohort had CRAB relapse compared to 35 patients (45%) in the maintenance cohort (hazard ratio: 0.71, 95% CI: 0.51-0.92, p=0.031). The type of end organ dysfunction at clinical relapse is shown in bar graphs in Fig. 2B.
Conclusions: Taken together, our results suggest that although maintenance therapy postpones disease relapse in the post-HSCT setting, it also impacts dynamics of the resurgence of secretory function within myeloma cells and shifts the nature of recurrence toward less biochemical relapse. Future studies will employ next generation sequencing prior to and during maintenance therapy combined with high sensitivity flow cytometry to identify the molecular basis of relapse. Genomics and cell surface markers may then more accurately predict relapse in MM patients on maintenance therapy.
Caimi:Genentech: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau. Malek:Amgen: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal